About Us
Message
Hi everyone! Thank you for your visiting our homepage. The new department of pharmacology launched on 1st of October in 2020.
The number of patients with neuropsychiatric disorders, including schizophrenia, dementia, and autism spectrum disorder is increasing rapidly in the past decades. We are addressing the machinery of the causes of such disorders. Most of these patients have problems with synaptic transmission. Synapses are the relay points for information transmitted within a complex neuronal network. Abnormal synaptic transmission is thought to be a cause of a neuropsychiatric disorder. In order to understand the machinery of the cause at the molecular level, we study disease model mice with biochemical and super-resolved fluorescence microscopies including STED microscopy and STORM. We will develop our research for drug discovery to cure patients suffering from neuropsychiatric disorders.
Gunma University is located in the very center of Japan and has good access from Tokyo. This university is surrounded by beautiful nature. From the window of our department, we can enjoy wonderful views of Mts. Akagi, Haruna, and Myogi. I am very looking forward to seeing how they will change throughout the four seasons. Gunma University has a long tradition as a neuroscience-oriented university for decades and been leading neuroscience research in Japan. Our university is one of a few universities fully equipped to perform cutting-edge neuroscience in Japan.
We are recruiting ambitious undergraduate and graduate students. Why don’t you join our department? Undergraduate students will learn the fun part of leading neuroscience in our department. We will teach and train graduate students so that they can work as genuine professional scientists. Your achievement in our department may be documented in a textbook and change the history of science. You can enjoy such excitement only in science.
I had been supervising a number of students in my previous working place, Max Planck Institute of Experimental Medicine in Germany. They have very diverse backgrounds, coming from Japan, Bulgaria, Turkey, Poland, Taiwan, India, Egypt, Serbia, and Germany. PhDs from my Max Planck group are working in top-level research institutions and universities as postdocs and group leaders. Based on my experience, I can and will supervise students from everywhere in the world. My dream is for PhDs from my group at Gunma University to develop their science all over the world in the future.
Contact me if you are interested in joining my group or if you want to know more details. My e-mail address is kawabe(at)gunma-u.ac.jp
Lab Members

Professor
Hiroshi Kawabe
I love doing good science and training students to be good scientists.

Associate Professor
Kenji Hanamura
I study the mechanism of neuronal development and function. My ultimate goals are understanding the pathophysiology of various neuropsychiatric disorders and developing therapeutic methods for the disorders.

Assistant Professor
Hiroyuki Yamazaki
I will do my best to contribute to the further development of science in Japan.

Assistant Professor
Noriko Koganezawa
I am very much interested in the mechanism of memory formation, especially at the synaptic level.
I love bench work and enjoy it every day to make a breakthrough in neuroscience.

Secretary
Tomoko Isono
I am organizing the lab so that lab members can concentrate on and enjoy their research.

Technical Assistant
Kazumi Kamiyama
I am doing my best to support daily experiments to develop science in the department.

Technical Assistant
Sachiko Tanaami
Lab works are a lot of fun, indeed. I am highly motivated to support research in the department.

Graduate Student
Mai Yamamura
Learning science in our department is a lot of fun. I proceed my project day by day to write manuscript in the next year.

Under Graduate Student
Banri Segawa
I will be a “two-way” professional; as a medical doctor and a neuroscientist!
For Students
How can I join the department of pharmacology?
There are three ways to join us.
- If you are an undergraduate student at Showa campus in Gunma University, just drop by after the lectures. There is already one student from the MD-PhD course working hard in our department.
- If you want to work in our department as a graduate student, take an exam for the master or the PhD course. We will welcome students from other countries.
- If you belong to a company or a university and if you want to work with us using setups in our department, you are also welcome. Just contact me (kawabe(at)gunma-u.ac.jp).
For the detailed admission guidelines (e.g. entrance exam), see the link below.
New information for the course starting from October will be updated in April. Note that the deadline for the application will be May/June. For the entrance, it will be required to pass an English exam or to have a good score at TOEFL or IELTS exams. The score will be valid for TOEFL or IELTS exams taken in the past 5 years.
Messages from a student

I have been enjoying the research at the department of pharmacology since the third grade of my medical course. In the first two years after entering Gunma University School of Medicine, I had no chance to do research although I was very interested in. I was working on neurobiology research before studying at this school of medicine. In order to make campus life more meaningful with my research experience, I joined the department of pharmacology. All department members are hardworking and friendly and I always feel at home at the department. In the MD-PhD course, I am working on an exciting study of imaging neurons in a living mouse brain using a two-photon microscope. The department of pharmacology is probably one of the best labs to do neuroscience in Japan; the department is using the cutting-edge super-resolution STED microscopy and the medical school has common research facilities equipped with a two-photon microscope, a super-resolution STORM microscope, and next-generation sequencers. If you are interested in joining this department, don’t hesitate to contact Hiroshi (kawabe(a)gunma-u.ac.jp, where (a) needs to be changed to @). He is a nice guy and will explain potential projects for you in case you want to join his department. I am looking forward to challenging exciting research together with you!
Research
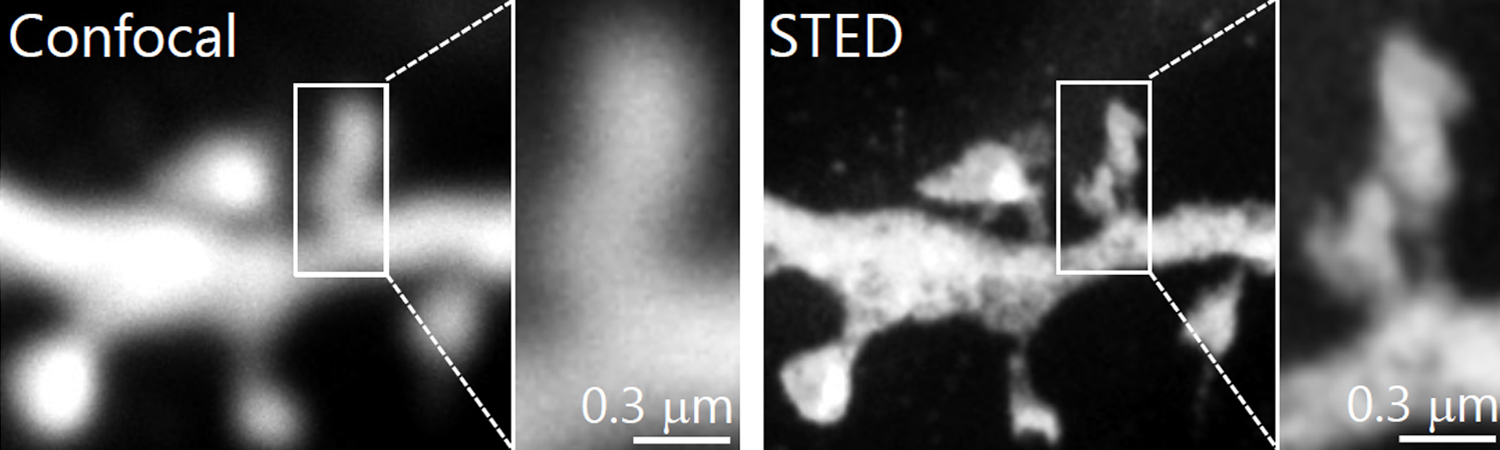
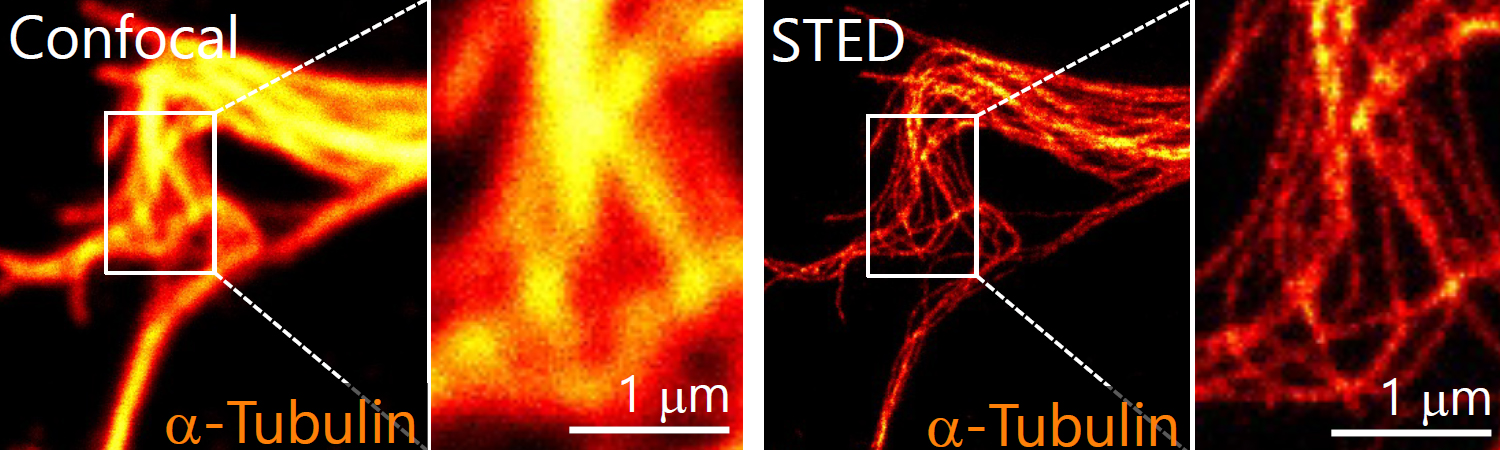
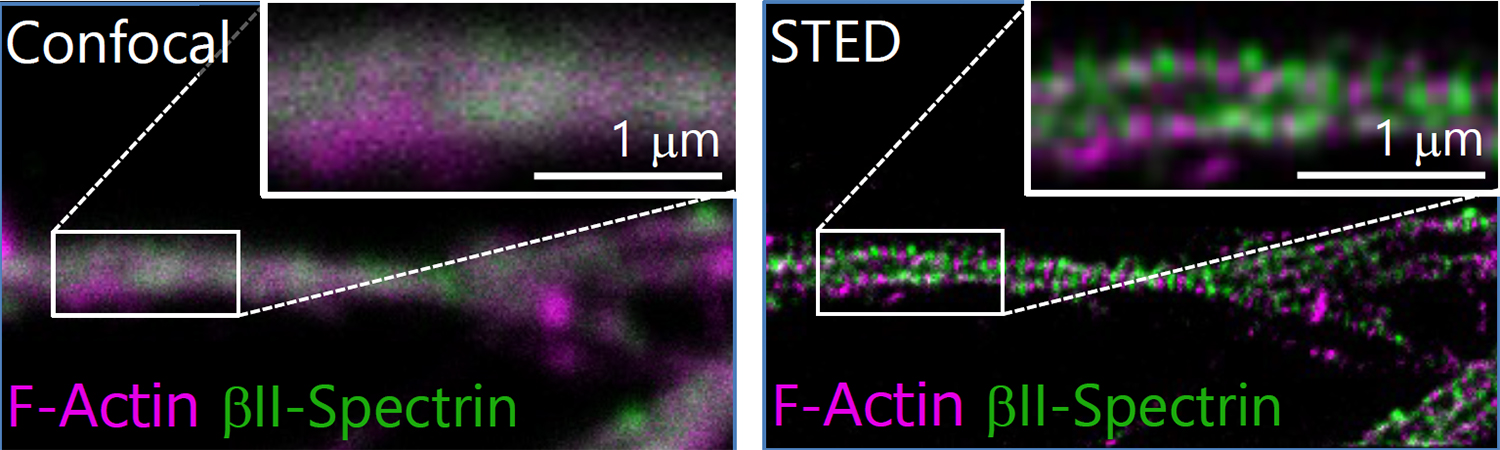
1. Morphological analysis using the STED microscopy
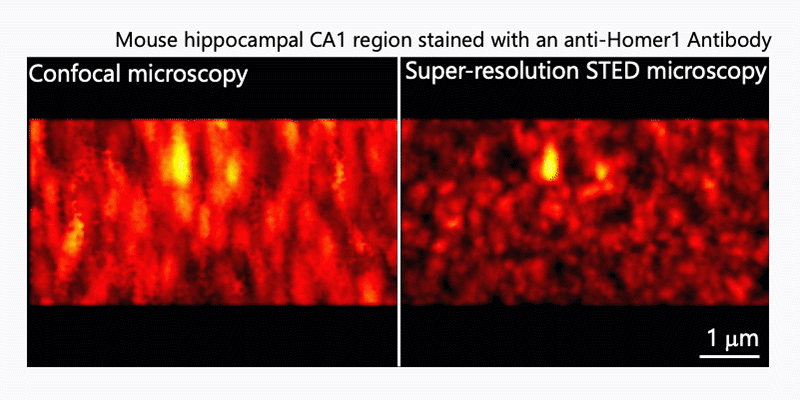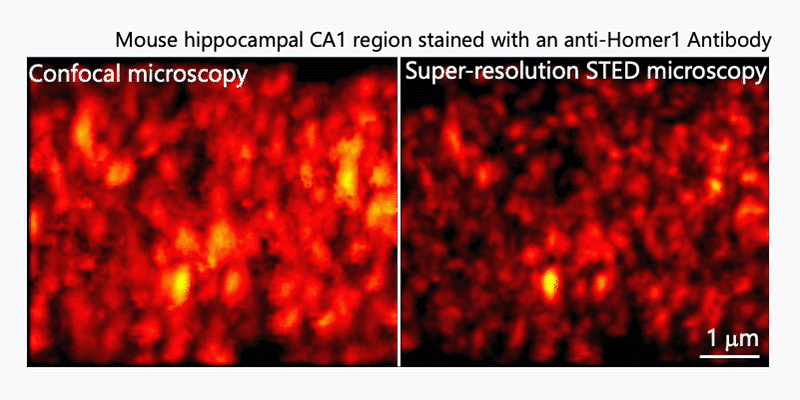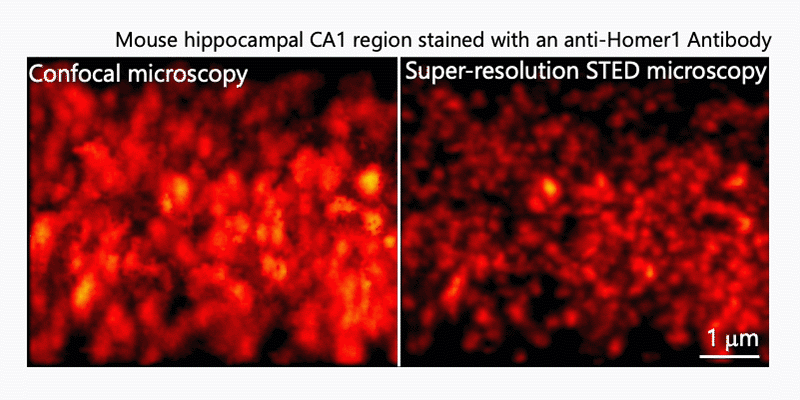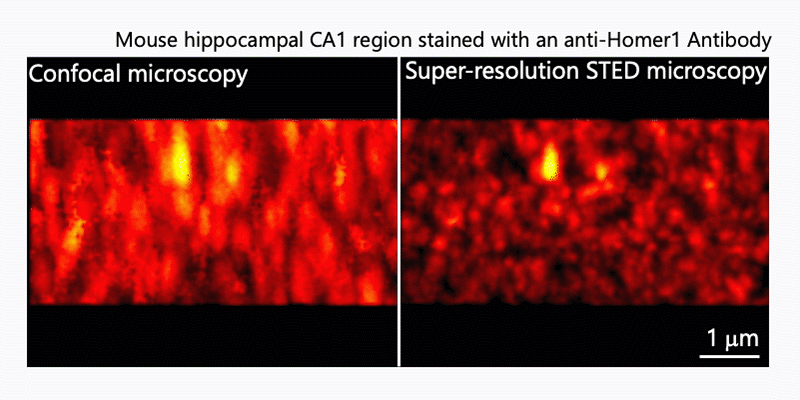
After immunohistochemistry, signals from a synaptic protein, Homer1 in mouse hippocampus were acquired by confocal (left) and super-resolution STED (right) microscopies. As shown in the right image, we can detect the structure and localization of each individual synapse by super-resolution STED microscopy using Homer1 as a marker of the synapse. There are limited number of microscopes that can resolve the precise localization of proteins in the brain tissue. We are developing the microscopic technology to further improve the resolution. Using STED microscopy, we will explore the mouse brain tissue to discover novel subcellular structures.
2. Molecular and genetical analysis of the causes of neuropsychiatric disorders
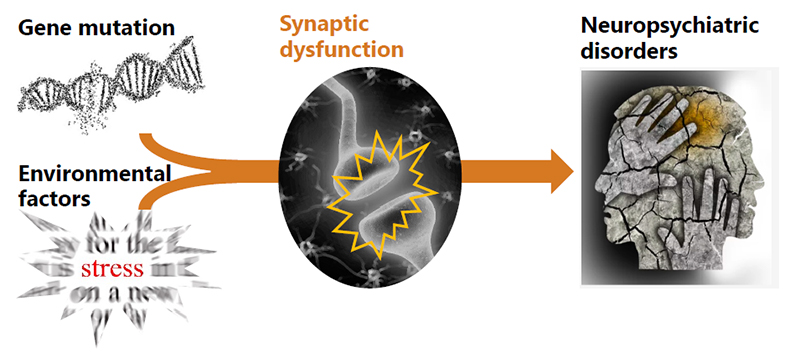
Recent genetic association studies demonstrate that certain gene mutations, as well as environmental factors (e.g. stress), could cause synaptic dysfunctions, leading to neuropsychiatric disorders. However, this model needs to be validated experimentally. We have already established mouse lines with mutations in genes involved in or related to neuropsychiatric disorders. We will analyze these mice to study the cause of neuropsychiatric disorders at the molecular level.
3. Biochemical and morphological analyses of synaptic protein degradation and its impact on cognitive function
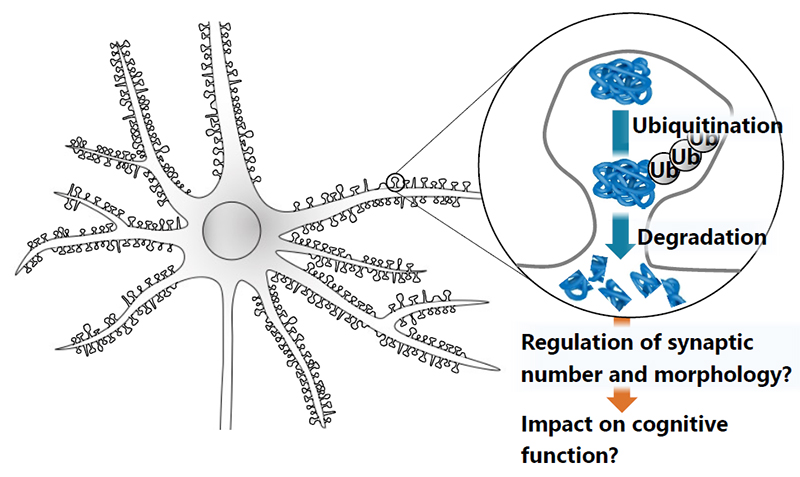
Synapse is composed of hundreds of proteins, each of which plays its own unique roles in synaptic transmission and synaptic plasticity. Most of synaptic proteins are ubiquitinated and targeted for protein degradation. However, it is not clear where and when proteins are degraded at the synapse. Also, it remains to be elucidated how synaptic protein degradation regulates the synaptic number and morphology. We will study them by biochemical methods and with STED microscopy to have an insight into its impact on cognitive functions. We will develop our study to discover drugs to improve cognitive function in the future.
Our collaborators
We had been collaborating internationally with colleagues in worldwide institutions listed below. Students and postdocs in our department would have a chance to visit them and perform experiments.
Germany
Max Planck Institute of Experimental Medicine, Max Planck Institute for Biophysical Chemistry, Berlin Charite University, University of Göttingen, Hamburg Eppedorf University, University of Lübeck, Leipzig University, Ulm University, RWTH Aachen University, Max Planck Institute of Immunobiology and Epigenetics, The Leibniz Institute for Neurobiology
Switzerland
ETH Zürich, University of Lausanne
France
Paris-Sorbonne University
UK
University of Dundee
Spain
University of Barcelona
Belgium
University of Liège
US
Harvard University, Stanford University, Thomas Jefferson University, University of Maryland, Northwestern University, Scripps institute, Salk Institute
Canada
University of Toronto, University of British Columbia
Australia
University of Adelaide
Brazil
University of Sao Paulo
China
Nanjing University, Tianjin Medical University
Singapore
National University of Singapore
Publication
Publication by Prof. Kawabe
- Koganezawa N, Hanamura K, Krueger-Burg D, Kawabe H. (2021) Super-resolved 3D-STED microscopy identifies a layer-specific increase in excitatory synapses in the hippocampal CA1 region of Neuroligin-3 KO mice. Biochemical and Biophysical Research Communications 582:144-149.
- Kawabe H. Stegmüller J. (2021) The role of E3 ubiquitin ligases in synapse function in the healthy and diseased brain. Molecular and cellular neurosciences 112:103602-103602.
- Leitz D.H.W, Duerr J, Mulugeta S, Seyhan Agircan A, Zimmermann S, Kawabe H. Dalpke A.H, Beers M.F, Mall M.A.(2021) Congenital Deletion of Nedd4-2 in Lung Epithelial Cells Causes Progressive Alveolitis and Pulmonary Fibrosis in Neonatal Mice International Journal of Molecular Sciences 22(11): 6146-6146.
- Ambrozkiewicz MC*, Borisova E, Schwark M, Ripamonti S, Schaub T, Smorodchenko A, Weber AI, Rhee HJ, Altas B, Yilmaz R, Mueller S, Piepkorn L, Horan ST, Straussberg R, Zaqout S, Jahn O, Dere E, Rosário M, Boehm-Sturm P, Borck G, Willig K, Rhee JS, Tarabykin V, Kawabe H.* (2021) The murine ortholog of Kaufman oculocerebrofacial syndrome protein Ube3b regulates synapse number by ubiquitinating Ppp3cc. Molecular Psychiatry 26(6):1980-1995. *=Corresponding authors
- Akter N, Fukaya R, Adachi R, Kawabe H and Kuba H.(2020) Structural and Functional Refinement of the Axon Initial Segment in Avian Cochlear Nucleus during Development.Journal of Neuroscience 40 (35): 6709-6721.
- Hoshino S, Kobayashi M, Tagawa R, Konno R, Abe T, Furuya K, Miura K, Wakasawa H, Okita N, Sudo Y, Mizunoe Y, Nakagawa Y, Nakamura T, Kawabe H, Higami Y. (2020) WWP1 knockout mice have exacerbated obesity-related phenotype in white adipose tissue but improved whole body glucose metabolism. FEBS Open Bio 10:306-315.
- Luo L, Ambrozkiewicz MC, Benseler F, Chen C, Dumontier E, Falkner S, Furlanis E, Gomez AM, Hoshina N, Huang WH, M Hutchison MA, Itoh-Maruoka Y, Lavery LA, Li W, Maruo T, Motohashi J, Pai ELL, Pelkey KA, Pereira A, Philips T, Sinclair JL, Stogsdill JA, Traunmüller L, Wang J, Wortel J, You W, Abumaria N, Beier KT, Brose N, Burgess HA, Cepko CL, Cloutier JF, Eroglu C, Goebbels S, Kaeser PS, Kay JN, Lu W, Luo L, Mandai K, McBain CJ, Nave KA, Prado MAM, Prado VF, Rothstein J, Rubenstein JLR, Saher G, Sakimura K, Sanes JR, Scheiffele P, Takai Y, Umemori H, Verhage M, Yuzaki M, Zoghbi HY, Kawabe H*, Craig AM*. (2020) Optimizing nervous system-specific gene targeting with Cre driver lines: prevalence of germline recombination and influencing factors. Neuron 106:1–29. *=Corresponding authors
- Ambrozkiewicz M.C, Cuthill K.J, Harnett D, Kawabe H, Tarabykin V. (2020) Molecular Evolution, Neurodevelopmental Roles and Clinical Significance of HECT-Type UBE3 E3 Ubiquitin Ligases. Cells 9(11).
- Ding X, Jo J, Wang C.Y, Cristobal C.D, Zuo Z, Ye Q, Wirianto M, Lindeke-Myers A, Choi J.Mi, Mohila C.A, Kawabe H, Jung S.Y, Bellen H.J, Yoo S.H, Lee H.K. (2020) The Daam2-VHL-Nedd4 axis governs developmental and regenerative oligodendrocyte differentiation. Genes & development 34(17-18):1177-1189.
- Duerr J, Leitz D.H.W, Szczygiel M, Dvornikov D, Fraumann S.G, Kreutz C, Zadora P.K, Seyhan Agircan A, Konietzke P, Engelmann T.A, Hegermann J, Mulugeta S, Kawabe H, Knudsen L, Ochs M, Rotin D, Muley T, Kreuter M, Herth F.J.F, Wielpütz M.O, Beers M.F, Klingmüller U, Mall M.A. (2020) Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nature communications 11(1):2012-2012.
- Ambrozkiewicz MC*, Schwark M, Kishimoto-Suga M, Borisova E, Hori K, Salazar-Lázaro A, Rusanova A, Altas B, Piepkorn L, Bessa P, Schaub T, Zhang X, Rabe T, Ripamonti S, Rosário M, Akiyama H, Jahn O, Kobayashi T, Hoshino M, Tarabykin V, Kawabe H*. (2018) Polarity acquisition in cortical neurons is driven by synergistic action of Sox9-regulated Wwp1 and Wwp2 E3 ubiquitin ligases and intronic miR-140. Neuron 100:1097-1115. *=Corresponding authors
- Ogawa M, Matsuda R, Tomokiyo M, Takada N, Yamamoto S, Shizukusihi S, Yamaji T, Yoshikawa Y, Yoshida M, Tanida I, Koike M, Murai M, Morita H, Takeyama H, Ryo A, Guan JL, Yamamoto M, Inoue JI, Yanagawa T, Fukuda M, Kawabe H, Ohnishi M. (2018) Molecular mechanisms of Streptococcus pneumoniae-targeted autophagy via pneumolysin, Golgi-resident Rab41, and Nedd4-1 mediated K63-linked ubiquitination. Cellular Microbiology 20:e12846.
- Bhouri M, Morishita W, Temkin P, Goswami D, Kawabe H, Brose N, Südhof TC, Craig AM, Siddiqui TJ, Malenka R. (2018) Deletion of LRRTM1 and LRRTM2 in adult mice impairs basal AMPA receptor transmission and LTP in hippocampal CA1 pyramidal neurons. Proc. Natl. Acad. Sci. USA. 115:E5382-E5389.
- Pei G, Buijze H, Liu H, Moura-Alves P, Goosmann C, Brinkmann V, Kawabe H, Dorhoi A, Kaufmann SHE. (2017) The E3 ubiquitin ligase NEDD4 enhances killing of membrane-perturbing intracellular bacteria by promoting autophagy. Autophagy 13:2041-2055.
- Henshall TL, Manning JA, Alfassy OS, Boase NA, Goel P, Kawabe H, Kumar S. (2017) Deletion of Nedd4-2 results in progressive kidney disease in mice. Cell Death Differ. 24:2150-2160.
- Lee JH, Jeon SA, Kim BG, Takeda M, Cho JJ, Kim DI, Kawabe H, Cho JY. (2017) Nedd4 deficiency in vascular smooth muscle promotes vascular calcification by stabilizing pSmad1. J. Bone Miner. Res. 32:927-938.
- Crosby JR, Zhao C, Jiang C, Bai D, Katz M, Greenlee S, Kawabe H, McCaleb M, Rotin D, Guo S, Monia BP. (2017) Inhaled ENaC antisense oligonucleotide ameliorates cystic fibrosis-like lung disease in mice. J. Cyst. Fibros. 16:671-680.
- Zhou Z, Kawabe H, Suzuki A, Shinmyozu K, Saga Y. (2017) NEDD4 controls spermatogonial stem cell homeostasis and stress response by regulating messenger ribonucleoprotein complexes. Nat. Commun. 8:15662.
- Sigler A, Oh WC, Imig C, Altas B, Kawabe H, Cooper BH, Kwon HB, Rhee JS, Brose N. (2017) Formation and maintenance of functional spines in the absence of presynaptic glutamate release. Neuron 94:304-311.
- Kawabe H, Mitkovski M, Kaeser PS, Hirrlinger J, Opazo F, Nestvogel D, Kalla S, Fejtova A, Verrier SE, Bungers SR, Cooper BH, Varoqueaux F, Wang Y, Nehring RB, Gundelfinger ED, Rosenmund C, Rizzoli SO, Südhof TC, Rhee JS, Brose N. (2017) ELKS1 localizes the synaptic vesicle priming protein bMunc13-2 to a specific subset of active zones. J. Cell Biol. 216:143-161.
- Jiang C, Kawabe H, Rotin D. (2017) The ubiquitin ligase Nedd4L regulates the Na/K/2Cl co-transporter NKCC1/SLC12A2 in the colon. J. Biol. Chem. 292:137-145.
- Ripamonti S, Ambrozkiewicz MC, Guzzi F, Gravati M, Biella G, Bormuth I, Hammer M, Tuffy LP, Sigler A, Kawabe H, Nishimori K, Toselli M, Brose N, Parenti M, Rhee JS. (2017) Transient oxytocin signaling primes the development and function of excitatory hippocampal neurons. elife 6:22466.
- Lu C, Thoeni C, Connor A, Kawabe H, Gallinger S, Rotin D. (2016) Intestinal knockout of Nedd4 enhances growth of Apcmin tumors. Oncogene 35: 5839- 5849.
- Canal M, Martín-Flores N, Pérez-Sisqués L, Romaní-Aumedes J, Altas B, Man HY, Kawabe H, Alberch J, Malagelada C. (2016) Loss of NEDD4 contributes to RTP801 elevation and neuron toxicity:implications for Parkinson’s disease. Oncotarget 7:58813-58831.
- Lipina TV, Prasad T, Yokomaku D, Luo L, Connor SA, Kawabe H, Wang YT, Brose N, Roder JC, Craig AM. (2016) Cognitive deficits in Calsyntenin-2-deficient mice associated with reduced GABAergic transmission. Neuropsychopharmacology 41:802-810.
- Ott C, Martens H, Hassouna I, Oliveira B, Erck C, Zafeiriou MP, Peteri UK, Hesse D, Gerhart S, Altas B, Kolbow T, Stadler H, Kawabe H, Zimmermann WH, Nave KA, Schulz-Schaeffer W, Jahn O, Ehrenreich H. (2015) Widespread expression of erythropoietin receptor in brain and its induction by injury. Mol. Med. 21:803-815.
- Hsia HE, Kumar R, Luca R, Takeda M, Courchet J, Nakashima J, Wu S, Goebbels S, An W, Eickholt B, Polleux F, Rotin D, Wu H, Rossner M, Bagni C, Rhee JS, Brose N, Kawabe H*. (2014) Ubiquitin E3 ligase Nedd4-1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth. Proc. Natl. Acad. Sci. USA. 111:13205-13210. *=Corresponding author
- Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, Rudenko G, Wang YT, Brose N, Craig AM. (2013) The specific α-Neurexin interactor Calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron 80:113-128.
- Siddiqui TJ, Tari PK, Conner SA, Zhang P, Dobie FA, She K, Kawabe H, Wang YT, Brose N, Craig AM. (2013) An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron 79:680-695.
- Nagpal P, Plant PJ, Correa J, Bain A, Takeda M, Kawabe H, Rotin D, Bain JR, Batt JA. (2012) The ubiquitin ligase Nedd4-1 participates in denervation-induced skeletal muscle atrophy in mice. PLOS One 7:e46427.
- Cooper B, Hemmerlein M, Ammermüller J, Imig C, Reim K, Lipstein N, Kalla S, Kawabe H, Brose N, Brandstätter JH, Varoqueaux F. (2012) Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J. Neurosci. 32:8040-8052.
- Kimura T*, Kawabe H*, Jiang C, Zhang W, Xiang YY, Lu C, Salter MW, Brose N, Lu WY, Rotin D. (2011) Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc. Natl. Acad. Sci. USA. 108:3216-3221. *=Equal contribution
- Kawabe H*, Neeb A, Dimova K, Young SM Jr, Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang DE, Umikawa M, Kariya K, Goebbels S, Nave KA, Rosenmund C, Jahn O, Rhee JS, Brose N*. (2010) Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron 65:358-372. *=Corresponding authors
- Fouladkou F, Lu C, Jiang C, Zhou L, She Y, Walls JR, Kawabe H, Brose N, Henkelman RM, Huang A, Bruneau BG, Rotin D. (2010) The ubiquitin ligase NEDD4-1 is required for heart development and is a suppressor of thrombospondin-1. J. Biol. Chem. 285:6770-6780.
- Gómez-Varela D, Kohl T, Schmidt M, Rubio ME, Kawabe H, Nehring RB, Schäfer S, Stühmer W, Pardo LA. (2010) Characterization of Eag1 channel lateral mobility in rat hippocampal cultures by single-particle-tracking with quantum dots. PLOS One 5:e8858.
- Trimbuch T, Beed P, Vogt J, Schuchmann S, Maier N, Kintscher M, Breustedt J, Schuelke M, Streu N, Kieselmann O, Brunk I, Laube G, Strauss U, Battefeld A, Wende H, Birchmeier C, Wiese S, Sendtner M, Kawabe H, Kishimoto-Suga M, Brose N, Baumgart J, Geist B, Aoki J, Savaskan NE, Bräuer AU, Chun J, Ninnemann O, Schmitz D, Nitsch R. (2009) Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell 138:1222-1235.
- Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, Stambolic V, Rotin D. (2008) The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc. Natl. Acad. Sci. USA. 105:8585-8590.
- Dimova K*, Kawabe H*, Betz A, Brose N, Jahn O. (2006) Characterization of the Munc13-calmodulin interaction by photoaffinity labeling. Biochim. Biophys. Acta Mol. Cell Res. 1763:1256-1265. *=Equal contribution
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. (2006) Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J. Biol. Chem. 281:19720-19731.
- Kawabe H, Sakisaka T, Yasumi M, Shingai T, Izumi G, Nagano F, Deguchi-Tawarada M, Takeuchi M, Nakanishi H, Takai Y. (2003) A novel rabconnectin-3-binding protein that directly binds a GDP/GTP exchange protein for Rab3A small G protein implicated in Ca2+-dependent exocytosis of neurotransmitter. Genes Cells 8:537-546.
- Nagano F*, Kawabe H*, Nakanishi H, Shinohara M, Deguchi-Tawarada M, Takeuchi M, Sasaki T, Takai Y. (2002) Rabconnectin-3, a novel protein that binds both GDP/GTP exchange protein and GTPase-activating protein for Rab3 small G protein family. J. Biol. Chem. 277:629-632. *=Equal contribution
- Kawabe H, Nakanishi H, Asada M, Fukuhara A, Morimoto K, Takeuchi M, Takai Y. (2001) Pilt, a novel peripheral membrane protein at tight junctions in epithelial cells. J. Biol. Chem. 276:48350-48355.
- Deguchi M, Iizuka T, Hata Y, Nishimura W, Hirao K, Yao I, Kawabe H, Takai Y. (2000) PAPIN. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/delta-catenin and p0071. J. Biol. Chem. 275:29875-29880.
- Kikyo M, Matozaki T, Kodama A, Kawabe H, Nakanishi H, Takai Y. (2000) Cell-cell adhesion-mediated tyrosine phosphorylation of nectin-2delta, an immunoglobulin-like cell adhesion molecule at adherens junctions. Oncogene 19:4022-4028.
- Yao I, Ohtsuka T, Kawabe H, Matsuura Y, Takai Y, Hata Y. (2000) Association of membrane-associated guanylate kinase-interacting protein-1 with Raf-1. Biochem. Biophys. Res. Commun. 270:538-542.
- Hirao K, Hata Y, Deguchi M, Yao I, Ogura M, Rokukawa C, Kawabe H, Mizoguchi A, Takai Y. (2000) Association of synapse-associated protein 90/ postsynaptic density-95-associated protein (SAPAP) with neurofilaments. Genes Cells 5:203-210.
- Hirao K, Hata Y, Yao I, Deguchi M, Kawabe H, Mizoguchi A, Takai Y. (2000) Three isoforms of synaptic scaffolding molecule and their characterization: multimerization between the isoforms and their interaction with N-methyl-D-aspartate receptors and SAP90/PSD-95-associated protein. J. Biol. Chem. 275:2966-2972.
- Kawabe H, Hata Y, Takeuchi M, Ide N, Mizoguchi A, Takai Y. (1999) nArgBP2, a novel neural member of ponsin/ArgBP2/vinexin family that interacts with synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP). J. Biol. Chem. 274:30914-30918.
- Shimizu K, Kawabe H, Minami S, Honda T, Takaishi K, Shirataki H, Takai Y. (1996) SMAP, an Smg GDS-associating protein having arm repeats and phosphorylated by Src tyrosine kinase. J. Biol. Chem. 271:27013-27017.
Publication by the former research group of the Department of Neurobiology and Behavior
- Yin XL, Jia QF, Zhang GY, Zhang JP, Shirao T, Jiang CX, Yin XY, Liu YS, Chen P, Gu XC, Qian ZK, Yin GZ, Sen Xia H, Hui L. (2019) Association between decreased serum TBIL concentration and immediate memory impairment in schizophrenia patients. Sci. Rep. 9:1622.
- Miki D, Kobayashi Y, Okada T, Miyamoto T, Takei N, Sekino Y, Koganezawa N, Shirao T, Saito Y. (2019) Characterization of functional primary cilia in human induced pluripotent stem cell-derived neurons. Neurochem. Res. 44:1736-1744.
- Mitsuoka T, Hanamura K, Koganezawa N, Kikura-Hanajiri R, Sekino Y, Shirao T. (2019) Assessment of NMDA receptor inhibition of phencyclidine analogues using a high-throughput drebrin immunocytochemical assay. J. Pharmacol. Toxicol. Methods. 106583
- Hanamura K, Koganezawa N, Kamiyama K, Tanaka N, Oka T, Yamamura M, Sekino Y, Shirao T. (2019) High-content imaging analysis for detecting the loss of drebrin clusters along dendrites in cultured hippocampal neurons. J. Pharmacol. Toxicol. Methods. 106607.
- Puspitasari A, Yamazaki H, Kawamura H, Nakano T, Takahashi A, Shirao T, Held KD. (2019) X-irradiation of developing hippocampal neurons causes changes in neuron population phenotypes, dendritic morphology and synaptic protein expression in surviving neurons at maturity. Neurosci. Res. S0168-0102: 30320-7.
- Miao S, Koganezawa N, Hanamura K, Puspitasari A, Shirao T. (2018) N-methyl-D-aspartate receptor mediates X-irradiation-induced drebrin decrease in hippocampus. Kitakanto Medical Journal 68: 111-115
- Hanamura K, Kamata Y, Yamazaki H, Kojima N, Shirao T. (2018) Isoform-dependent regulation of drebrin dynamics in dendritic spines. Neuroscience 379: 67–76
- Yamazaki H, Sasagawa Y, Yamamoto H, Bito H, Shirao T. (2018) CaMKIIβ is localized in dendritic spines as both drebrin-dependent and drebrin-independent pools. J. Neurochem. 146:145-159.
- Yasuda H, Kojima N, Yamazaki H, Hanamura K, Sakimura K, Shirao T. (2018) Drebrin isoforms critically regulate NMDAR- and mGluR-dependent LTD induction. Front. Cell. Neurosci. 12:330.
- 小金澤紀子、花村健次、白尾智明 (2017) 「ヒトiPS細胞由来神経細胞を用いた医薬品評価系の現状について」日本薬理学雑誌 149: 104-109
- Shirao T, Hanamura K, Koganezawa N, Ishizuka Y, Yamazaki H, Sekino Y. (2017) The role of drebrin in neurons. J. Neurochem. 141:819-834.
- Koganezawa N, Hanamura K, Sekino Y, Shirao T. (2017) The role of drebrin in dendritic spines. Mol. Cell. Neurosci. 84:85-92.
- Li B, Ding S, Feng N, Mooney N, Ooi YS, Renf L, Diep J, Kelly MR, Yasukawa LL, Patton JT, Yamazaki H, Shirao T, Jackson PK, Greenberg HB. (2017) Drebrin restricts rotavirus entry by inhibiting dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 114:E3642-E3651.
- Kajita Y, Kojima N, Koganezawa N, Yamazaki H, Sakimura K, Shirao T. (2017) Drebrin E regulates neuroblast proliferation and chain migration in the adult brain. Eur. J. Neurosci. 46:2214-2228.
- Hanamura K, Washburn HR, Sheffler-Collins SI, Xia NL, Henderson N, Tillu DV, Hassler S, Spellman DS, Zhang G, Neubert TA, Price TJ, Dalva MB. (2017) Extracellular phosphorylation of a receptor tyrosine kinase controls synaptic localization of NMDA receptors and regulates pathological pain. PLoS Biol. 15:e2002457.
- Shirao T, Sekino Y. (Eds.) (2017) Drebrin. (Advances in Experimental Medicine and Biology, vol 1006) Tokyo, Springer
- Sekino Y, Koganezawa N, Mizui T, Shirao T. (2017) Role of Drebrin in Synaptic Plasticity. Adv. Exp. Med. Biol. 1006:183-201.
- Yamazaki H, Shirao T. (2017) Homer, Spikar, and Other Drebrin-Binding Proteins in the Brain. Adv. Exp. Med. Biol. 1006:249-268.
- Shirao T, Koganezawa N, Yamazaki H, Hanamura K, Imamura K. (2017) Localization of Drebrin: Light Microscopy Study. Adv. Exp. Med. Biol. 1006:105-118.
- Shirao T, Sekino Y. (2017) General Introduction to Drebrin. Adv. Exp. Med. Biol. 1006:3-22.
- Ishizuka Y, Hanamura K. (2017) Drebrin in Alzheimer’s Disease. Adv. Exp. Med. Biol. 1006:203-223.
- Hanamura K. (2017) Drebrin in Neuronal Migration and Axonal Growth. Adv. Exp. Med. Biol. 1006:141-155.
- Puspitasari A, Koganezawa N, Ishizuka Y, Kojima N, Tanaka N, Nakano T, Shirao T. (2016) X Irradiation Induces Acute Cognitive Decline via Transient Synaptic
- Dysfunction. Radiat. Res. 185:423-30.
- Fujieda T, Koganezawa N, Ide Y, Shirao T, Sekino Y. (2015) An Inhibitory Pathway Controlling the Gating Mechanism of the Mouse Lateral Amygdala Revealed by Voltage-Sensitive Dye Imaging. Neuroscience Letters 590:126-131.
- Xu S, Buraschi S, Morcavallo A, Genua M, Shirao T, Peiper SC, Gomella LG, Birbe R, Belfiore A, Iozzo RV, Morrione A. (2015) A novel role for drebrin in regulating progranulin bioactivity in urothelial cancer. Oncotarget 6:10825-39.
- Ishizuka Y, Shimizu H, Takagi E, Kato M, Yamagata H, Mikuni M, Shirao T. (2014) Histone deacetylase mediates the decrease in drebrin cluster density induced by amyloid beta oligomers. Neurochem. Int. 76:114-121.
- Tanabe K, Yamazaki H, Inaguma Y, Asada A, Kimura T, Takahashi J, Taoka M, Ohshima T, Furuichi T, Isobe T, Nagata K, Shirao T, Hisanaga S. (2014) Phosphorylation of drebrin by cyclin-dependent kinase 5 and its role in neuronal migration. PLOS One 9:e92291.
- Ishikawa M, Shiota J, Ishibashi1 Y, Hakamata T, Shoji S, Fukuchi M, Masaaki Tsuda M, Shirao T, Sekino Y, Baraban JM, Tabuchi A. (2014) Cellular localization and dendritic function of rat isoforms of the SRF coactivator MKL1 in cortical neurons. NeuroReport 25:585-592.
- Mizui T,Sekino Y, Yamazaki H, Ishizuka Y, Takahashi H, Kojima N, Kojima M, Shirao T. (2014) Myosin Ⅱ ATPase activity mediates the long-term potentiation-induced exodus of stable F-actin bound by drebrin A from dendritic spines. PLOS One 9: e85367.
- Yamazaki H, Kojima N, Kato K, Hirose H, Iwasaki T, Mizui T, Takahashi H, Hanamura K, Roppongi RT, Koibuchi N, Sekino Y, Mori N, Shirao T. (2013) Spikar, a novel drebrin-binding protein, regulates the formation and stabilization of dendritic spines. J. Neurochem. 128:507-522.
- 白尾智明、山崎博幸 (2013)「樹状突起棘(スパイン)のアクチンフィラメントと微小管」 Clinical Neuroscience 31: 1302-1395.
- Ishikawa M, Shiota J, Ishibashi Y, Hakamata T, Shoji S, Fukuchi M, Tsuda M, Shirao T, Sekino Y, Ohtsuka T, Baraban JM, Tabuchi A. (2013) Identification, expression and characterization of rat isoforms of the SRF coactivator MKL1. FEBS Open Bio. 3:387-393.
- Ishizuka Y, Kakiya N, Witters LA, Oshiro N, Shirao T, Nawa H, Takei N. (2013) AMP-activated protein kinase (AMPK) counteractsbrain-derived neurotrophic factor (BDNF)-induced mammalian target of rapamycin complex 1 (mTORC1) signaling in neurons. J. Neurochem. 127:66-77.
- Shirao T, Gonzalez-Billault C. (2013) Actin filaments and microtubules in dendritic spines. J. Neurochem. 126:155-164.
- Roppongi RT, Kojima N, Hanamura K, Yamazaki H, Shirao T. (2013) Selective reduction of drebrin and actin in dendritic spines of hippocampal neurons by activation of 5-HT2A receptors. Neurosci. Lett. 547:76-81.
- Shirai K, Mizui T, Suzuki Y, Okamoto M, Hanamura K, Yoshida Y, Hino M, Noda S, AL-Jahdari WS, Chakravarti A, Shirao T, Nakano T. (2012) X-Irradiation Changes Dendritic Spine Morphology and Density through Reduction of Cytoskeletal Proteins in Mature Neurons. Radiation Research 179:630-636.
- Canto CB, Koganezawa N, Beed P, Moser EI, Witter MP. (2012) All layers of medial entorhinal cortex receive presubicular and parasubicular inputs. J. Neurosci. 32: 17620-17631.
- Kato K, Shirao T, Yamazaki H, Imamura K, Sekino Y. (2012) Regulation of AMPA receptor recruitment by the actin binding protein drebrin in cultured hippocampal neurons. J. Neurosci. Neuroeng. 1: 153-160.
- Tanaka K, Sato K, Yoshida T, Fukuda T, Hanamura K, Kojima N, Shirao T, Yanagawa T, and Watanabe H. (2011) Evidence for cell density affecting C2C12 myogenesis: possible regulation of myogenesis by cell-cell communication. Muscle and Nerve 44:968-977.
- Kobayashi-Yamazaki C, Shirao T, Sasagawa Y, Maruyama Y, Akita H, Saji M, Sekino Y. (2011) Lesions of the supramammillary nucleus decrease self-grooming behavior of rats placed in an open field The Kitakanto Medical Jorunal 61:287-292.
- Han W, Takamatsu Y, Yamamoto H, Kasai S, Endo S, Shirao T, Kojima N, Ikeda K. (2011) Inhibitory role of inducible cAMP early repressor (ICER) in methamphetamine-induced locomotor sensitization. PLOS One 6:e21637.
- Okamoto T, Endo S, Shirao T, Nagao S. (2011) Role of Cerebellar Cortical Protein Synthesis in Transfer of Memory Trace of Cerebellum-Dependent Motor Learning. J. Neurosci. 31:8958-66.
- Kambe T, Motoi Y, Inoue R, Kojima N, Tada N, Kimura T, Sahara N, Yamashita S, Mizoroki T, Takashima A, Shimada K, Ishiguro K, Mizuma H, Onoe H, Mizuno Y, Hattori N. (2011) Differential regional distribution of phosphorylated tau and synapse loss in the nucleus accumbens in tauopathy model mice. Neurobiol. Dis. 42:404-414.
- Kaminuma T, Suzuki Y, Shirai K, Mizui T, Noda S, Yosida Y, Funayama T, Takahasi T, Kobayashi Y, Shirao T, Nakano T. (2010) Effectiveness of carbon-ion beams for apoptosis induction in rat primary immature hippocampal neurons. J. Rad. Res. 51:627-631.
- Hanamura K, Mizui T, Kakizaki T, Roppongi TR, Yamazaki H, Yanagawa Y, Shirao T (2010) Low accumulation of drebrin at glutamatergic postsynaptic sites on GABAergic neurons. Neuroscience 169:1489-1500.
- Perez-Martinez M, Gordon-Alonso M, Cabrero JR, Barrero-Villar M, Rey M, Mittelbrunn M, Lamana A, Morlino G, Calabia C, Yamazaki H, Shirao T, Vazquez J, Gonzalez-Amaro R, Veiga E, Sanchez-Madrid F. (2010) F-actin-binding protein drebrin regulates CXCR4 recruitment to the immune synapse. J. Cell Sci. 123:1160-1170.
- Mercer JC, Mottram LF Qi Q, Lee YC, Bruce D, Iyer A, Yamazaki H, Shirao T, Choe MH, Peterson BR, August A. (2010) Chemico-Genetic Identification of Drebrin as a Regulator of Calcium Responses. Int. J. Biochem. Cell Biol. 42:337-345.
- Kojima N, Hanamura K, Yamazaki H, Ikeda T, Itohara S, Shirao T. (2010) Genetic disruption of the alternative splicing of drebrin gene impairs context-dependent fear learning in adulthood. Neuroscience 165: 138-150.
- Okamoto M, Suzuki Y, Shirai K, Mizui T, Yoshida Y, Noda S, Al-Jahdari WS, Shirao T, Nakano T. (2009) Effect of Irradiation on the Development of Immature Hippocampal Neurons In Vitro. Radiat. Res. 172:718-724.
- Aoki C, Kojima N, Sabaliauskas N, Shah L, Oakford J, Ahmed T, Yamazaki H, Hanamura K, Shirao T. (2009) Drebrin A Knock-Out Eliminates the Rapid Form of Homeostatic Synaptic Plasticity at Excitatory Synapses of Intact Adult Cerebral Cortex. J. Comp. Neurol. 517:105-121.
- Ito M, Shirao T, Doya K, Sekino Y. (2009) Three-dimensional distribution of Fos-positive neurons in the supramammillary nucleus of the rat exposed to novel environment. Neurosci. Res. 64:397-402.
- Mizui T, Kojima N, Yamazaki H, Katayama M, Hanamura K, Shirao T. (2009) Drebrin E is involved in the mechanism regulating axonal growth through actin-myosin interactions. J. Neurochem. 109:611-622.
- Takahashi T, Yamazaki H, Hanamura K, Sekino Y, Shirao T. (2009) AMPA receptor inhibition causes abnormal dendritic spines by destabilizing drebrin. J. Cell. Sci. 122:1211-1229.
- Ivanov A, Esclapez M, Pellegrino1 C, Shirao T, Ferhat L. (2009) Drebrin A regulates the dendritic spine plasticity and synaptic function in cultured hippocampal neurons. J. Cell. Sci. 122: 524-534.
- Kojima N, Borlikova G, Sakamoto T, Yamada K, Ikeda T, Itohara S, Niki H, Endo S. (2008) Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory. J. Neurosci. 28:6459-6472.
- Song M, Kojima N, Hanamura K, Sekino Y, Inoue KH, Mikuni M, Shirao T. (2008) Expression of drebrin E in migrating neuroblasts in adult rat brain: coincidence between drebrin E disappearance from cell body and cessation of migration. Neuroscience 152:670-682.
- Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T. (2007) Drebrin A content correlates with spine head size in the adult mouse cerebral cortex. J. Comp. Neurol. 503:618-26.
- Sekino Y, Kojima N, Shirao T. (2007) Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem. Int. 51:92-104.
- Kato K, Sekino Y, Takahashi H, Yasuda H, Shirao T. (2007) Increase of AMPA receptors-mediated miniature EPSC amplitude after chronic NMDA receptor blockade in cultured hippocampal neurons. Neurosci. Lett. 418: 4-8.
- Kojima N, Shirao T. (2007) Synaptic dysfunction and disruption of the postsynaptic drebrin-actin complex: the study of neurological disorders accompanied by cognitive deficits. Neurosci. Res. 58: 1-5.
- Majoul I, Shirao T, Sekino Y, Duden R. (2007) Many faces of Drebrin: from building dendritic spines and stabilizing gap junctions to shaping neurite-like cell processes. Histochem. Cell. Biol. 127: 355-361.
- Shirai K, Mizui T, Suzuki Y, Kobayashi Y, Nakano T, Shirao T. (2006) Differential effects of x-irradiation on immature and mature hippocampal neurons in vitro. Neurosci. Lett. 399: 57-60.
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. (2006) AMPA receptor downscaling at the onset of Alzheimer’s pathology in double knock-in mice. Proc. Natl. Acad. Sci. USA. 103: 3410-3415.
- Fujisawa S, Shirao T, Aoki C. (2006) In vivo, competitive blockade of NMDA receptors induces rapid shape change of post-synaptic spines and F-actin reorganization within dendritic spines of adult rat cortex. Neuroscience 140:1177-1187.
- Takahashi H, Mizui T, Shirao T. (2006) Downregulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurons. J. Neuochem. 97(s1):110-115.
- Sekino Y, Tanaka S, Hanamura K, Yamazaki H, Sasagawa Y, Xue Y, Hayashi K, Shirao T. (2006) Activation of N-methyl-D-aspartate receptor induces a shift of drebrin distribution: disappearance from dendritic spines and appearance in dendritic shafts. Mol. Cell. Neurosci. 31: 493-504.
- Mahadomrongkul V, Huerta PT, Shirao T, Aoki C. (2005) Stability of the distribution of spines containing drebrin A in the sensory cortex layer I of mice expressing mutated APP and PS1 genes. Brain Res. 1064: 66-74.
- Mizui T, Takahashi H, Sekino Y, Shirao T. (2005) Overexpression of drebrin A in immature neurons induces the accumulation of F-actin and PSD-95 into dendritic filopodia, and the formation of large abnormal protrusions. Mol. Cell. Neurosci. 30: 149-157.
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. (2005) Drebrin A is a Postsynaptic Protein that Localizes in vivo to the Submembranous Surface of Dendritic Sites Forming Excitatory Synapses. J. Comp. Neurol. 483:383-402.
- Link to the former department of neurobiology and behavior:http://neuro.dept.med.gunma-u.ac.jp/?page_id=96&lan=en